Search This Blog
Tuesday 29 January 2013
Why Are Humans Made Of Carbon? Chemist Points To Electrons, Molecular Bonds
Everything on earth is made up of combinations of different elements –
all of which can be found on the periodic table. Considering that the
periodic table contains 118 elements it seems a pity that organic life
tends to feature only five or six of those elements in any vast
quantities. The main one being carbon.
As well as being the main element in organic matter, carbon atoms are
the only element in both graphite and diamond.
It would be impossible for life on earth to exist without carbon. Carbon
is the main component of sugars, proteins, fats, DNA, muscle tissue,
pretty much everything in your body. The reason carbon is so special is
down to the electron configuration of the individual atoms. Electrons
exist in concentric ‘shells’ around the central nucleus and carbon has
four electrons in its outermost shell. As the most stable thing for an
atom to have is eight electrons, this means that each carbon can form
four bonds with surrounding atoms.
Credit: http://www.huffingtonpost.com
Monday 28 January 2013
Chemistry of Cooking
WHAT ARE ACIDS AND BASES? An acid is defined as a solution with more positive hydrogen ions than negative hydroxyl ions, which are made of one atom of oxygen and one of hydrogen. Acidity and basicity are measured on a scale called the pH scale. The value of freshly distilled water is seven, which indicates a neutral solution. A value of less than seven indicates an acid, and a value of more than seven indicates a base. Common acids include lemon juice and coffee, while common bases include ammonia and bleach.
WHY DOES FOOD SPOIL? Processing and improper storage practices can expose food items to heat or oxygen, which causes deterioration. In ancient times, salt was used to cure meats and fish to preserve them longer, while sugar was added to fruits to prevent spoilage. Certain herbs, spices and vinegar can also be used as preservatives, along with anti-oxidants, most notably Vitamins C and E. In processed foods, certain FDA-approved chemical additives also help extend shelf life.
This report has been produced thanks to a generous grant from the Camille and Henry Dreyfus Foundation, Inc.
Reported January 2009
Saturday 26 January 2013
Thursday 24 January 2013
Blog's Highlight
Wednesday 23 January 2013
Statistical Mechanics
Defining statistical mechanics: Statistical Mechanics provies the connection
between microscopic motion of individual atoms of matter and
macroscopically observable properties such as temperature, pressure,
entropy, free energy,
heat capacity, chemical potential, viscosity, spectra, reaction rates, etc.
- Statistical Mechanics provides the microscopic basis for thermodynamics, which, otherwise, is just a phenomenological theory.
- Microscopic basis allows calculation of a wide variety of properties not dealt with in thermodynamics, such as structural properties, using distribution functions, and dynamical properties - spectra, rate constants, etc., using time correlation functions.
- Because a statistical mechanical formulation of a problem begins with a detailed microscopic description, microscopic trajectories can, in principle and in practice, be generated providing a window into the microscopic world. This window often provides a means of connecting certain macroscopic properties with particular modes of motion in the complex dance of the individual atoms that compose a system, and this, in turn, allows for interpretation of experimental data and an elucidation of the mechanisms of energy and mass transfer in a system.
For more details and lecture notes click here.
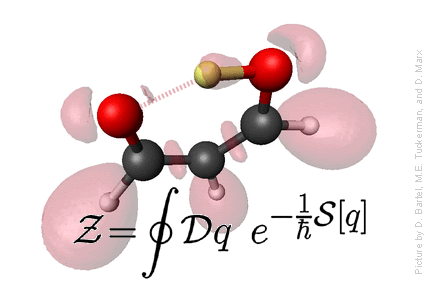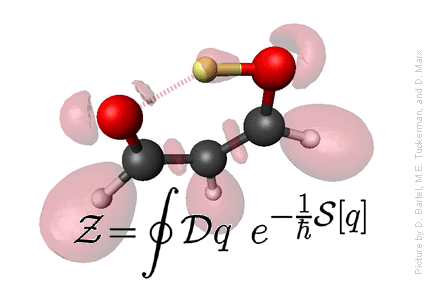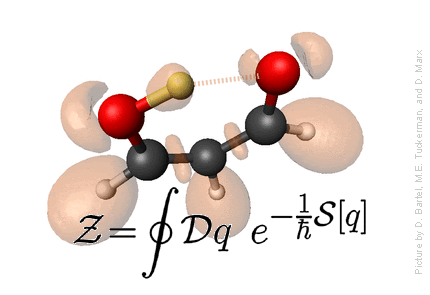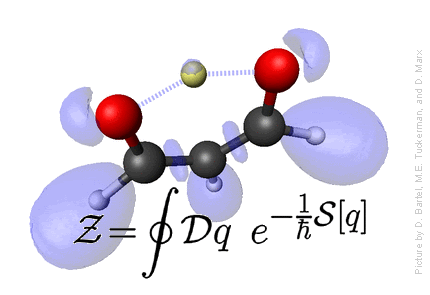
Credit: Professor M. Tuckerman
New 2-D Material for Next Generation High-Speed Electronics
Scientists at CSIRO and RMIT University have produced a new
two-dimensional material that could revolutionise the electronics
market, making “nano” more than just a marketing term.
The material – made up of layers of crystal known as molybdenum
oxides – has unique properties that encourage the free flow of electrons
at ultra-high speeds.
In a paper published in the January issue of materials science journal Advanced Materials, the researchers explain how they adapted a revolutionary material known as graphene to create a new conductive nano-material. For more details click here.
 |
| Artist impression of high carrier mobility through layered molybdenum oxide crystal lattice. (Credit: Dr Daniel J White, ScienceFX) |
Credit: Dr Daniel J White, http://www.csiro.au
Monday 21 January 2013
Discovery Opens up Possibilities for a New Generation of Targeted Therapies for Cancer.
In 1953, Cambridge researchers Watson and Crick published a
paper describing the interweaving ‘double helix’ DNA structure – the
chemical code for all life.
Now, in the year of that scientific landmark’s 60th
Anniversary, Cambridge researchers have published a paper proving that
four-stranded ‘quadruple helix’ DNA structures – known as G-quadruplexes
– also exist within the human genome. They form in regions of DNA that
are rich in the building block guanine, usually abbreviated to ‘G’. For more click here.
 |
| Credit: http://www.cam.ac.uk |
Credit: Prof. Shankar Balasubramanian, University of Cambridge
Atomic Chemistry
Atom,
smallest unit of a chemical element that can exist. In ancient Greek philosophy
the word “atom” was used to describe the smallest bit of matter that could
be conceived of. This “fundamental particle”, to use the present-day term
for this concept, was thought of as indestructible; in fact, the Greek word for
atom (atomos) means “not divisible”.
Knowledge about the size and nature of
the atom did not begin to be acquired until long after the beginnings of
experimental science in the 16th and 17th centuries. Although many of the new
“experimental philosophers” believed in the reality of atoms, the progress
of science owed little to the idea. The first quantitative explanation of the
behaviour of matter in terms of atoms was attempted by Daniel Bernoulli in 1738,
but his work was largely ignored. However, chemistry was discovering things
about matter that only the idea of atoms could explain.
Chemists recognized that
all liquids, gases, and solids can be broken down into their ultimate
components, or elements. For example, salt is a chemical compound formed when
the elements sodium and chlorine react together and become joined in an intimate
form known as a chemical compound. Air, by contrast, was found to consist of a
mixture of the gases nitrogen and oxygen, which do not react with each other.
Credit: library.thinkquest.org/
Sunday 20 January 2013
Saturday 19 January 2013
Overview of Computational Chemistry
Chemists have been some of the most active and innovative participants in this
rapid expansion of computational science. Computational chemistry is simply
the application of chemical, mathematical and computing skills to the solution
of interesting chemical problems. It uses computers to generate information
such as properties of molecules or simulated experimental results. Some common
computer software used for computational chemistry includes:
- Gaussian xx, Gaussian 09 currently
- GAMESS
- MOPAC
- Spartan
- Sybyl
Click here for more details.
Credit: http://www.shodor.org/chemviz/overview/
Friday 18 January 2013
Chemistry of Glass
Obsidian, a black volcanic glass, is probably the best known of the naturally occurring glasses. It was used by early man to form cutting tools, arrowheads and spearheads and is now used by modern man to make the sharpest surgical blades.
Synthetic glass was originally prepared by heating a mixture of sodium oxide (or sodium carbonate), calcium oxide and silicon dioxide (sand). If calcium oxide was not added to the melt, soda glass was obtained. Pure soda glass is not usable because of its high solubility in water. Soda lime glass has a large coefficient of expansion when heated and a low resistance to the effects of acids and bases. It usually has a green color due to the presence of iron oxide in the sand. It was later discovered that this color could be removed by adding manganese oxide to the melt when a colorless glass was desired.
Manufactured glass is presumed to have been first used as a glaze for pottery. The earliest known glaze is on stone beads of the Badarian age of Egypt. These beads ranked in value with precious metals and stones at the time! The Egyptians first made vessels out of glass by the laborious process in which the glass was applied over a wooden or metal rod bit by bit. A cylinder of light blue glass made by this method dates back to the Akkad dynasty in 2600 B.C. Glass was first pressed into open molds in 1200 B.C. There is some evidence that Mesopotamia was the location where glass was first manufactured.
For more details click here.
Synthetic glass was originally prepared by heating a mixture of sodium oxide (or sodium carbonate), calcium oxide and silicon dioxide (sand). If calcium oxide was not added to the melt, soda glass was obtained. Pure soda glass is not usable because of its high solubility in water. Soda lime glass has a large coefficient of expansion when heated and a low resistance to the effects of acids and bases. It usually has a green color due to the presence of iron oxide in the sand. It was later discovered that this color could be removed by adding manganese oxide to the melt when a colorless glass was desired.
Manufactured glass is presumed to have been first used as a glaze for pottery. The earliest known glaze is on stone beads of the Badarian age of Egypt. These beads ranked in value with precious metals and stones at the time! The Egyptians first made vessels out of glass by the laborious process in which the glass was applied over a wooden or metal rod bit by bit. A cylinder of light blue glass made by this method dates back to the Akkad dynasty in 2600 B.C. Glass was first pressed into open molds in 1200 B.C. There is some evidence that Mesopotamia was the location where glass was first manufactured.
For more details click here.
Credit: Prof. Richard Banks, http://chemistry.boisestate.edu
Wednesday 16 January 2013
Dye Chemistry
Look around you, at your clothes, the walls, the floor. Chances are that you see before you a riot of colour. Humans have been fascinated by colour for thousands of years and use colours to warn, to seduce and primarily to decorate. So, what about the chemistry behind the decoration? What makes one molecule coloured, and another not? Why do some clothes fade in the wash?
The chemical basis of colours is the reason many people choose to do chemistry. The basis of this project is the chemistry behind fabric dyes- what are they? What are the origins of dyes? How are they made? What affects the way they attach to different fibres?
The search for highly coloured, colour fast dyes has fuelled major industry from ancient times right up to the present, from the Roman dye factories at Tyre, to modern chemical companies such as ICI. Nowadays, the chemist with a knowledge of organic chemistry is at the forefront of new dye development, altering the structures of known dyes, and inventing new ones.
Credit: http://www.chm.bris.ac.uk/webprojects2002The chemical basis of colours is the reason many people choose to do chemistry. The basis of this project is the chemistry behind fabric dyes- what are they? What are the origins of dyes? How are they made? What affects the way they attach to different fibres?
The search for highly coloured, colour fast dyes has fuelled major industry from ancient times right up to the present, from the Roman dye factories at Tyre, to modern chemical companies such as ICI. Nowadays, the chemist with a knowledge of organic chemistry is at the forefront of new dye development, altering the structures of known dyes, and inventing new ones.
Tuesday 15 January 2013
Lecture on Environmental Chemistry
Environmental chemistry is that branch of chemical science that deals with the production, transport, reactions, effects, and fates of chemical species in the water, air, terrestrial, and biological environment and the effects of human activities thereon. For more click here.
Credit: http://www.asdlib.org/onlineArticle
Monday 14 January 2013
Graphene-derived Nanorings of Electronic Power
Computational Chemistry Highlights: Graphene-derived nanorings of electronic power: P. V. Avramov, D. G. Fedorov, P. B. Sorokin, S. Sakai, S. Entani, M. Ohtomo, Y. Matsumoto, H. Naramoto, J. Phys. Chem. Lett. 3 (2012) 2003-2...
Credit: http://www.compchemhighlights.org
Sunday 13 January 2013
Largest Known Structure in the Universe
An international team of astronomers, led by academics from the University of Central Lancashire (UCLan), has found the largest known structure in the universe. The large quasar group (LQG) is so large that it would take a vehicle travelling at the speed of light some 4 billion years to cross it. The team publish their results in the journal Monthly Notices of the Royal Astronomical Society. For more details click here.
Credit: Royal Astronomical SocietySaturday 12 January 2013
Walking Molecules!
Nanomotors are used throughout biology to perform tasks. Spectacular
examples include the motor protein myosin that makes muscles contract by "walking" along molecular tracks in the cell.
Professor David
Leigh's group have made the first synthetic walking molecules that move
directionally along molecular tracks. The ultimate goal of
such research is to produce artificial molecular vehicles that
can transport cargoes and perform other complex tasks at the nanoscale.
However, such 'molecular
engineering' is not easy: at the molecular level gravity is too
weak to hold the walkers onto tracks and special molecular glue,
footholds and attachment points all
have to be carefully designed to make a successful walking
molecule (see video).
Credit: Professor David Leigh and co-workers, University of Manchester
Friday 11 January 2013
Thursday 10 January 2013
Stars are responsible for forging every heavy element in the universe
when they fuse hydrogen and when they explode at the ends of their
lives. But they also create a strange third type of chemical bond
between atoms, caused by their incredible magnetic fields. This
previously unknown type of bond could lead to new research in quantum
science, perhaps even quantum computing. For more click here.
 |
| White Dwarf Stars This image from the Hubble Space Telescope shows a close-up of ancient white dwarf stars in the Milky Way. NASA and H. Richer (University of British Columbia) |
Credit: http://www.popsci.com/science/article. Article you can find here.
Wednesday 9 January 2013
What is EPR Spectroscopy?
Electron spin resonance (ESR) spectroscopy, also referred to as electron
paramagnetic resonance (EPR) spectroscopy, is a versatile,
nondestructive analytical technique which can be used for a variety of
applications including: oxidation and reduction processes, biradicals
and triplet state molecules, reaction kinetics, as well as numerous
additional applications in biology, medicine and physics. However, this
technique can only be applied to samples having one or more unpaired
electrons. For more details click here.
 |
| Image Credit: http://web.nmsu.edu |
Credit: http://epr.cm.utexas.edu
Tuesday 8 January 2013
A Tribute to Stephen Hawking - The Man of the Universe
Stephen Hawking is the former Lucasian Professor of Mathematics at the University of Cambridge and author of A Brief History of Time
which was an international bestseller. Now Director of Research at the
Centre for Theoretical Cosmology at Cambridge, his other books for the
general reader include A Briefer History of Time, the essay collection Black Holes and Baby Universe and The Universe in a Nutshell. For more details click here.
Credit: http://www.hawking.org.uk/
Credit: http://www.hawking.org.uk/
Monday 7 January 2013
Introduction to Mössbauer Spectroscopy: Part 1
The technique of Mössbauer spectroscopy is widely used in mineralogy to
examine the valence state of iron, which is found in nature as Fe0 (metal), Fe2+, and Fe3+,
as well as the type of coordination polyhedron occupied by iron atoms
(trigonal, tetrahedral, octahedral, etc.). It is sometimes used to
determine redox ratios in glasses and (less successfully) in rocks.
Mössbauer spectroscopy is also used to assist in the identification of
Fe oxide phases on the basis of their magnetic properties. For more details click here.
Credit: M. Darby Dyar, Department of Astronomy, Mount Holyoke College
Sunday 6 January 2013
Chemistry - Facts
It is vital that the next generation understands that chemistry is
everywhere; is a transforming force that improves lives and our society;
and is an important profession, because careers in chemistry offer
opportunities to change the world.
.jpg) |
| Image Credit: http://hilary-gio.blogspot.in |
Credit: ACS, Chemistry for Life
Saturday 5 January 2013
Air Pollution
Air pollution is the presence in the atmosphere of any substance at a
concentration great enough to produce an undesirable effect on humans,
animals, vegetation, or materials, or to significantly alter the natural
balance of any ecosystem. Air pollutants can be solids, liquids, or gases,
and can have local, regional, and global impacts.
Friday 4 January 2013
Introduction to Supramolecular Chemistry
The emergence of supramolecular chemistry has had a profound effect on how efficiently chemists prepare structures of different sizes and shapes with dimension in the range of 1 to 100 nm using spontaneous secondary interactions such as hydrogen bonding, dipoledipole, charge transfer, van der Waals, and p-p stacking interactions. This so-called “bottom up” approach to construct nanostructures is advantageous over the “top down” approach such as microlithography which requires substantial effort to fabricate microstructures and devices as the target structures are extended to the range below 100 nm. For more details click here.
 |
| Image credit: Professor
Kenneth N. Raymond UC Berkeley Chancellor's Professor |
Credit: http://scholar.lib.vt.edu
Thursday 3 January 2013
What is Laser?
The term laser should not sound alien to you. We often encounter
laser in our daily life, examples include the laser pointer used in classrooms,
and the CD-ROMs in a computer or in a hi-fi that are used to read the data
stored in a CD. In industry, laser is often used for cutting and microscopic
processing. For military purposes, laser is used to intercept guided missiles.
Scientists have also accurately measured the distance between the Earth and
the Moon by using laser; the error involved is only a few centimeters. These
are some extensive applications of laser. So actually how is it produced?
We will explain the basic principles of laser below. For more details click here.
Credit: http://www.hk-phy.org
Wednesday 2 January 2013
Particle in a 1-D Box
For detailed derivation click here.
 |
| Image credit: http://chemwiki.ucdavis.edu |
Tuesday 1 January 2013
Subscribe to:
Posts (Atom)
- Do not get over excited over happiness and do not get over depressed over sorrow.
- Do not get over bonded with anyone and anybody because it can lead to problems and sorrow.
- Never think that my duty is the topmost or lowermost. Every duty is respectful. The responsibility undertaken or given as per the position is the noblest duty.
- Elevate yourselves, family, society and nation and never denigrate yourselves, family, society and nation.
- We are our own closest relatives and if not properly utilised we will become our closest enemies.
- There are possibilities of success and failure in any endeavour. One cannot assure success always.
- Death is inevitable for everyone in this world. In any endeavour at the maximum an individual may die.
- People may say good and also they may say bad. Approach them with stabilised mind.
- Take anything after scientifically, logically and rationally analysing them.
- Perform your duty, responsibility and accept the privileges eligible for you.
- First change ourselves and then try to change others.
- We are all instruments /tools in the hands of the nature for performing the duty. So do not think that I am doing the duty. Think that I am an instrument to do the duty.
- Results of action may not be sweet always. Accept what ever may be the result.
- Follow the path of great scholars who guided the world. Listen their messages.
- Results and rewards will come and go but stick to your duty with devotion, dedication and sincerity.