,"I am sure the air in heaven must be this wonder working gas of delight". So wrote poet Robert Southey of nitrous oxide, N2O,
also known as nitrogen oxide, dinitrogen monoxide, hyponitrous acid
anhydride and facticious air. However, its most well known name is
'laughing gas' due to its intoxicating effects when inhaled. For more details click here.
Courtesy: Dr. Ewan Cameron and Dr. Paul May, School of Chemistry, University of Bristol
Courtesy: Dr. Ewan Cameron and Dr. Paul May, School of Chemistry, University of Bristol




















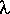 = 2d sin
= 2d sin