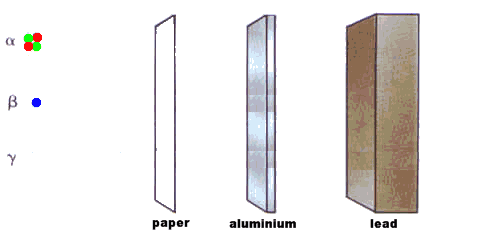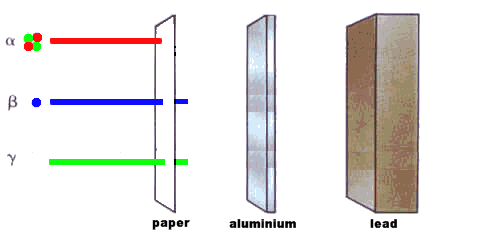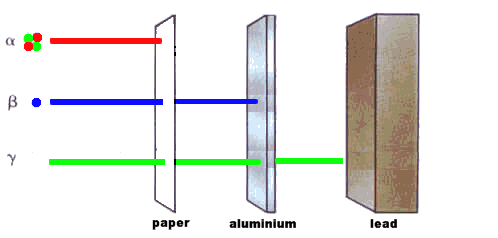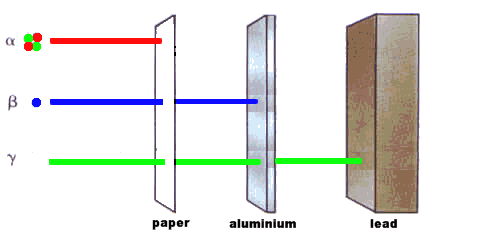
Cement would make a useful material for locking away radioactive waste
except for the fact that conventional cement is susceptible to
weathering. When water infiltrates its pores, it freezes and when it
thaws, the resulting cracks can fracture the cement blocks. Moreover,
adding foreign materials to the standard cement slows the hydration
process required for the mix to harden leading to greater porosity and
an increased risk of radioactive elements leaching out through long-term
wear and tear.
Read more here...
Read more here...
 |
| Credit: http://www.iucr.org |